Infectious Diseases In-Vitro Diagnostics Market Size, Statistics, Trend Analysis and Forecast Report, 2020 - 2027
Infectious Diseases in Vitro Diagnostics market is segmented by Product (Instruments, Reagents), Technology (Immunoassay, Clinical Chemistry, Molecular Diagnostics, Hematology, Urinalysis), Application (Diabetes, Oncology, Cardiology, Nephrology), End User (Laboratories [Large/Reference Laboratories, Medium-sized Laboratories, Small Laboratories] Hospitals, Academics, Point-Of-Care Testing, Patient Self-Testing) and Region (United States, Canada, Mexico, France, Germany, Italy, Spain, United Kingdom, Russia, China, India, Philippines, Malaysia, Australia, Austria, South Korea, Middle East, Japan, Africa and Rest of World)
- Report ID : MD1683 |
- Pages : 200 |
- Tables : 88 |
- Formats :
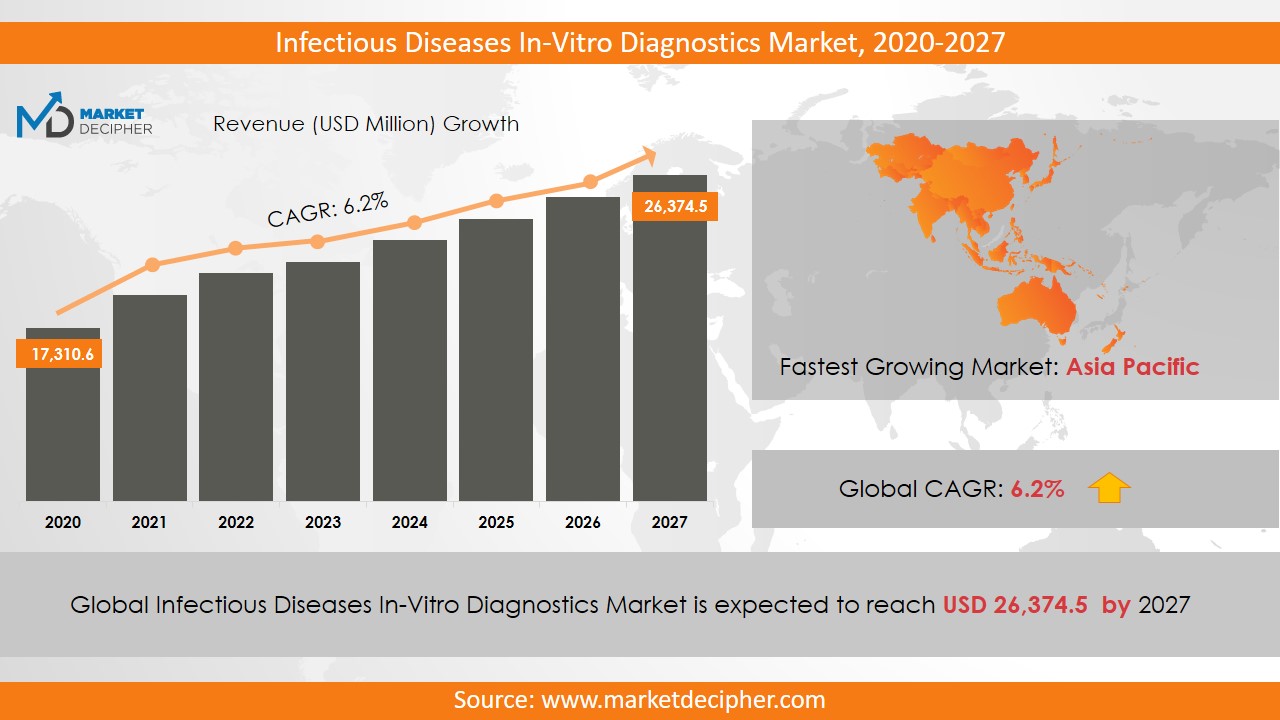
Infectious Diseases In-Vitro Diagnostics Market size was estimated at $70,332.4 Million in 2019 and is expected to reach $97,652.7 Million by 2027, growing at a CAGR of 4.8% during the forecast period of 2020 to 2027.
Infectious diseases are caused when microorganisms, including fungus, parasite, bacteria, and virus attack the body. They are usually transmitted from one person to another quite easily. This is when an infectious disease in-vitro diagnostics is required to identify the potential agent responsible for such infection so that doctors can determine the appropriate amount of treatments for the patient. Currently, the world is under the threat of a deadly pandemic, COVID-19, which makes the market for such diagnostics quite paramount.
Infectious Diseases in Vitro Diagnostics Market Growth Factors
According to the WHO, millions of people have been infected with the ill-effects of Corona Virus. It is estimated that many more cases are yet to be confirmed. To stop the spread, early diagnosis is required, which gives the infectious disease in vitro diagnostics market upward rise in the scales for the coming years. If market analysts are to be believed, the market is supposed to be at its peak by the year 2027. Not only India but also the global critical players including Bio-Rad Laboratories Inc, Becton Dickinson and Company, and Abbott Laboratories are some of the major influencers of this market.
Infectious Diseases in Vitro Diagnostics Market Segmentation
Infectious Diseases in Vitro Diagnostics Market Country Analysis
The value of the global infectious diseases in-vitro diagnostics market stood at $16.3 billion in 2019 and is expected to record a CAGR of 6.2% between 2020 and 2027. The market is dominated by industrially developed countries in North America and Europe due to favorable government policies and the availability of funds for research and development activities. Promising markets of Asia-Pacific regions are seeing dramatic growth, as well. Rapid population growth, continuous increase in awareness, and heavy investments in healthcare facilities and infrastructure are the key factors that are fueling the growth of this market in the Asia Pacific. The companies that are driving the infectious diseases in-vitro diagnostics market include Abbot Laboratories, Gen-Probe, Inc, Roche Diagnostics, and Luminex Corporation.
Infectious Diseases in Vitro Diagnostics Market Share and Competition
Key companies operating in this industry are: Roche Diagnostics Limited (Switzerland), Siemens Healthineers (Germany), Danaher Corporation (US), Abbott Laboratories (US), Thermo Fisher Scientific (US), Johnson & Johnson (US), Becton, Dickinson and Company (US), Bio-Rad Laboratories (US), Sysmex Corporation (Japan), bioMérieux (France), Diasorin (Italy), Ortho-Clinical Diagnostics (US), and Qiagen (Germany).
Report Highlights
• Historical data available (as per request)
• Estimation/projections/forecast for revenue and unit sales (2020 – 2027)
• Data breakdown for every market segment (2020 – 2027)
• Gross margin and profitability analysis of companies
• Price analysis of each product type
• Business trend and expansion analysis
• Import and export analysis
• Competition analysis/market share
• Supply chain analysis
• Client list and case studies
• Market entry strategy
Industry Segmentation and Revenue Breakdown
Product & Service Analysis (Revenue, USD Million, 2020 - 2027)
• Reagents & Kits
• Instruments
o Fully-automated Instruments
o Semi-automated Instruments
o Others
• Data Management Software
• Services
Technology Analysis (Revenue, USD Million, 2020 - 2027)
• Immunochemistry/Immunoassay
o Enzyme-Linked Immunosorbent Assay (ELISA)
Chemiluminescence Immunoassay (CLIA)
Fluorescence Immunoassay (FIA)
Colorimetric Immunoassay (CI)
o Radioimmunoassays (RIA)
o Rapid Tests
o Western Blot
o ELISPOT
• Clinical Chemistry
o Basic Metabolic Panel
o Electrolyte Panel
o Liver Panel
o Lipid Profile
o Renal Profile
o Thyroid Function Panel
o Specialty Chemicals
• Molecular Diagnostics
o Polymerase Chain Reaction (PCR)
o Isothermal Nucleic Acid Amplification Technology (INAAT)
o Hybridization (In-situ Hybridization & FISH)
o DNA Sequencing & NGS
o Microarray
o Others
• Microbiology
• Hematology
• Coagulation & Hemostasis
• Urinalysis
• Others
Application Analysis (Revenue, USD Million, 2020 - 2027)
• Diabetes
• Infectious Diseases
• Oncology/Cancer
• Cardiology
• Nephrology
• Autoimmune Diseases
• Drug Testing/Pharmacogenomics
• HIV/AIDS
• Others
End User Analysis (Revenue, USD Million, 2020 - 2027)
• Laboratories
o Large/Reference Laboratories
o Medium-sized Laboratories
o Small Laboratories
• Hospitals
• Academics
• Point-Of-Care Testing
• Patient Self-Testing
• Others
Region Analysis (Revenue, USD Million, 2020 - 2027)
• United States
• Canada
• Mexico
• France
• Germany
• Italy
• Spain
• United Kingdom
• Russia
• China
• India
• Philippines
• Malaysia
• Australia
• Austria
• South Korea
• Middle East
• Japan
• Africa
• Rest of World
Infectious Diseases in Vitro Diagnostics Market Companies
• Roche Diagnostics Limited (Switzerland)
• Siemens Healthineers (Germany)
• Danaher Corporation (US)
• Abbott Laboratories (US)
• Thermo Fisher Scientific (US)
• Johnson & Johnson (US)
• Becton, Dickinson and Company (US)
• Bio-Rad Laboratories (US)
• Sysmex Corporation (Japan)
• bioMérieux (France)
• Diasorin (Italy)
• Ortho-Clinical Diagnostics (US)
• Qiagen (Germany).
Available Versions:-
United States Infectious Diseases in Vitro Diagnostics Market Industry Research Report
Europe Infectious Diseases in Vitro Diagnostics Market Industry Research Report
Asia Pacific Infectious Diseases in Vitro Diagnostics Market Industry Research Report
In-vitro diagnostics is crucial for making advancements in health care as these help the physicians to monitor every stage of a particular disease. This industry has led to great discoveries and effects of various viruses like HIV, HBV, and HCV. The advancements in this market has led to the expansion of successful management of a disease in every stage. The market constitutes of microbiology testing, immunochemistry testing, infectious disease testing and other diagnostics. The market is all set to achieve new heights in the coming years.
The advancement of technology has led to the introduction of portable machines for testing. As the number of target and infectious diseases increases the market to expand more and more. This market helps the medical specialists to discover new ways of treatment and precautions. The government bodies also provide funds to this sector as this market serves as a measure of development in healthcare. If a particular region has a wider market of In-vitro diagnostics then it is assumed to be developed and up to date in medical terms. The key drivers of this market are the population of age in a particular region. As a person ages, he or she is more likely to suffer from one or are infectious or chronic diseases. The development of R&D and government initiatives also help this market to grow further. Due to lack of proper financial support, budget and reduction in reimbursements, this market notices a steep decrease in its share.
J Mitra & Co., India’s leading global company in In-Vitro Diagnostics, has announced on 17 June 2020, the launch of its new test kit Total Antibody Detection Elisa Test Kit. “This is a completely indigenous kit - an Atmanirbhar initiative with R&D done inhouse, and sourcing done from Indian manufacturers and suppliers. In keeping with the Governments Make in India initiative, we have created the most robust supply chain ecosystem for material sourcing as well as for supplying to various government agencies as per the demands.”, informed the Chairman of J Mitra & Co., Lalit Mahajan.
Celltrion Group, a giant provider for In-Vitro Diagnostics tests, has announced that it is going to launch a Point-of-Care Antigen Testing (POCT) Kit in partnership with BBB on 16 June 2020. This Covid-19 rapid antibody testing kit has a sensitivity of up to 95%. The In-Vitro Diagnostics have reached a new level of research after the launch of many testing kits due to the novel Covid-19. “We have successfully built solid partnerships with local device companies that are prominent in the field of diagnostic testing.”, Celltrion said.
PURCHASE OPTIONS
20% Free Customization ON ALL PURCHASE
*Terms & Conditions Apply
Looking for report on this market in a particular region or country? Get In Touch
Request Free Sample
Please fill in the form below to Request for free Sample Report
-
Office Hours Mon - Sat 10:00 - 16:00
-
Call Us +91 6201075429
-
Send Us Mail sales@marketdecipher.com

Market Decipher is a market research and consultancy firm involved in provision of market reports to organisations of varied sizes; small, large and medium.
© 2018 Market Decipher. All Rights Reserved



